Centrifugation - Definition, Principle, Types, Handling Procedure, Applications
- Centrifugation is a technique used for the separation of particles from a solution according to their size, shape, density, viscosity of the medium and rotor speed.
- The particles are suspended in a liquid medium and placed in a centrifuge tube. The tube is then placed in a rotor and spun at a define speed.
- Separation through sedimentation could be done naturally with the earth gravity, nevertheless, it would take ages. Centrifugation is making that natural process much faster.
- Rotation of the rotor about a central axis generates a centrifugal force upon the particles in the suspension.
Definition of Centrifugation
It is a unit operation working for separation separating the
consequent present in a dispersion with the help of centrifugal force for
example centrifugal force includes the earth revolves around the sun. It is a
technique which involves the application of centrifugal force to separate
particles from a solution according to their size, shape, density, the
viscosity of the medium and rotor speed.
Principle of Centrifugation
- In a solution, particles whose density is higher than that of the solvent sink (sediment), and particles that are lighter than it floats to the top.
- The greater the difference in density, the faster they move. If there is no difference in density (isopycnic conditions), the particles stay steady.
- To take advantage of even tiny differences in density to separate various particles in a solution, gravity can be replaced with the much more powerful “centrifugal force” provided by a centrifuge.
- A centrifuge is a piece of equipment that puts an object in rotation around a fixed axis (spins it in a circle), applying a potentially strong force perpendicular to the axis of spin (outward).
- The centrifuge works using the sedimentation principle, where the centripetal acceleration causes denser substances and particles to move outward in the radial direction.
- At the same time, objects that are less dense are displaced and move to the center.
- In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low- density substances rise to the top.
Types of Centrifuge
LOW-SPEED CENTRIFUGE
- Most laboratories have a standard low-speed centrifuge used for routine sedimentation of heavy particles
- The low-speed centrifuge has a maximum speed of 4000-5000rpm
- These instruments usually operate at room temperatures with no means of temperature control.
- Two types of rotors are used in it, Fixed angle and Swinging bucket.
- It is used for sedimentation of red blood cells until the particles are tightly packed into a pellet and supernatant is separated by decantation.
HIGH-SPEED CENTRIFUGES
- High-speed centrifuges are used in more sophisticated biochemical applications, higher speeds and temperature control of the rotor chamber are essential.
- The high-speed centrifuge has a maximum speed of 15,000 – 20,000 RPM
- The operator of this instrument can carefully control speed and temperature which is required for sensitive biological samples.
- Three types of rotors are available for high-speed centrifugation- Fixed angle, Swinging bucket and Vertical rotors
ULTRACENTRIFUGES
- It is the most sophisticated instrument.
- Ultracentrifuge has a maximum speed of 65,000 RPM (100,000’s x g).
- Intense heat is generated due to high speed thus the spinning chambers must be refrigerated and kept at a high vacuum.
- It is used for both preparative work and analytical work.
Types of Centrifugation
Differential Pelleting (differential centrifugation)
- It is the most common type of centrifugation employed.
- Tissue such as the liver is homogenized at 32 degrees in a sucrose solution that contains buffer.
- The homogenate is then placed in a centrifuge and spun at constant centrifugal force at a constant temperature.
- After some time a sediment forms at the bottom of a centrifuge called pellet and an overlying solution called supernatant.
- The overlying solution is then placed in another centrifuge tube which is then rotated at higher speeds in progressing steps.
Density Gradient Centrifugation
- This type of centrifugation is mainly used to purify viruses, ribosomes, membranes, etc.
- A sucrose density gradient is created by gently overlaying lower concentrations of sucrose on higher concentrations in centrifuge tubes
- The particles of interest are placed on top of the gradient and centrifuge in ultracentrifuges.
- The particles travel through the gradient until they reach a point at which their density matches the density of surrounding sucrose.
- The fraction is removed and analyzed.
Rate-Zonal Density-Gradient Centrifugation
- Zonal centrifugation is also known as band or gradient centrifugation
- It relies on the concept of sedimentation coefficient (i.e. movement of sediment through the liquid medium)
- In this technique, a density gradient is created in a test tube with sucrose and high density at the bottom.
- The sample of protein is placed on the top of the gradient and then centrifuged.
- With centrifugation, faster-sedimenting particles in sample move ahead of slower ones i.e. sample separated as zones in the gradient.
- The protein sediment according to their sedimentation coefficient and the fractions are collected by creating a hole at the bottom of the tube.
- Isopynic Centrifugation
- The sample is loaded into the tube with the gradient-forming solution (on top of or below pre-formed gradient, or mixed in with self-forming gradient)
- The solution of the biological sample and cesium salt is uniformly distributed in a centrifuge tube and rotated in an ultracentrifuge.
- Under the influence of centrifugal force, the cesium salts redistribute to form a density gradient from top to bottom.
- Particles move to point where their buoyant density equals that part of gradient and form bands. This is to say the sample molecules move to the region where their density equals the density of gradient.
- It is a “true” equilibrium procedure since depends on bouyant densities, not velocities
Eg: CsCl, NaI gradients for macromolecules and nucleotides – “self-forming” gradients under centrifugal force.
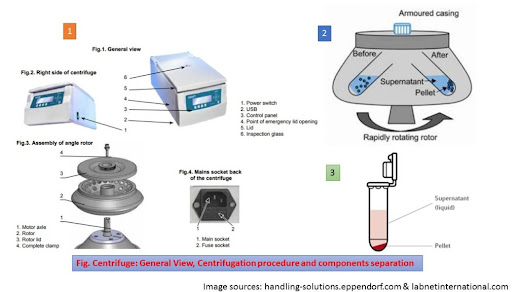
Proper Handling Procedure Centrifugation
- Ensure that the centrifuge tubes are properly balanced and that the speed and tube length is in accordance with the tube and the centrifuge manufacturer’s recommendations.
- Do not use tubes that are not properly sized for the rotor. If using a swinging-bucket rotor, ensure that the tubes are placed in accordance with the manufacturer’s instructions; long tubes (e.g., greater than 100 mm) placed in the corner tube holders closest to the rotor shaft may break when the rotor buckets swing out.
- For centrifuges with swinging-bucket rotors, fasten a protective inner safety lid (if available for your model centrifuge) onto the bucket; for those with fixed-angle rotors, fasten an inner safety lid to the rotor before centrifugation.
- Ensure that the safety lid is properly sealed and positively locked into place.
- Ensure that the rotor has completely stopped spinning before opening the lid, even if an “open lid” indicator lights and the safety interlock disengages. In some cases, the rotor may not be visible; therefore, the user should allow a reasonable amount of time for the rotor to stop and should feel the top of the lid for the cessation of vibration before opening it.
- Never attempt to stop a moving rotor with your hands or with a tool or object (e.g., a paper towel).
- All personnel should follow universal precautions when performing centrifugation and other functions that may expose workers to splashed blood or body fluids. These precautions include personal protective equipment like wearing gloves, facial protection (e.g., face shields), gowns or laboratory coats, and plastic aprons; these are described in detail in the Clinical and Laboratory Standards Institute (CLSI) tentative guideline and the Occupational Safety and Health Administration’s (OSHA) bloodborne pathogens standard.
- Spillage and break of tubes should be considered as the blood-borne pathogen hazard.
- Cleanliness minimizes the possible spread of infections.
- Speed of centrifuge should be checked once in 3 months while timer to be checked per week
Uses of Centrifuge
In the clinical laboratory, a centrifuge is applicable for
the following purposes and they are:
- Remove cellular elements from blood to provide cell-free plasma or serum for analysis.
- Remove chemically precipitated protein from an analytical specimen.
- Separate protein-bound from free ligand in immunochemical and another assay.
- Separation of the subcellular organelle, DNA, RNA.
- Extract solutes in biological fluids from aqueous to organic solvents.
- Separate lipid components.
Applications of Centrifugation
- To separate two miscible substances
- To analyze the hydrodynamic properties of macromolecules
- Purification of mammalian cells
- Fractionation of subcellular organelles (including membranes/membrane fractions) Fractionation of membrane vesicles
- Separating chalk powder from water
- Removing fat from milk to produce skimmed milk
- Separating particles from an air-flow using cyclonic separation
- The clarification and stabilization of wine
- Separation of urine components and blood components in forensic and research laboratories
- Aids in the separation of proteins using purification techniques such as salting out, e.g. ammonium sulfate precipitation.
Safety precautions during handling Centrifuge
The following points should take in mind during the
operation of the centrifuge ( some points may vary according to the type of
centrifuge)-
For safety reasons, inspections of the centrifuge are
carried out by the authorized service at least once a year after the period of
warranty. The reason for more frequent inspections could be a
corrosion-inducing environment. Examinations should end with issuing a report
of validation that checks on the technical state of the laboratory centrifuge.
It is being recommended to establish a document where every repair and review
are being registered. Both these documents should be stored in the place of use
of the centrifuge.
Inspection procedure carried out by the operator
- The user has to pay special attention to the fact that the centrifuge parts of key importance due to safety reasons are not damaged. This remark is specifically important as for:
- Centrifuge accessories and especially structural changes, corrosion, preliminary cracks, abrasion of metal parts.
- Inspection of the rotor assembly.
- Inspection of bio seals of the buckets if such are used.
- Control of execution of the guarantee yearly technical inspection of the centrifuge
- It is not allowed to lift or shift the centrifuge during operation and rest on it.
- It is not allowed to stay in the safety zone within 30 cm distance around the centrifuge neither leave within this zone some things, e.g. glass vessels.
- It is not allowed to put any objects on the centrifuge.
Cover opening
- The cover opening isn’t allowed to open the cover manually in the emergency procedure when the rotor is still turning
Rotor
- The rotor is not allowed to use the rotors and round carriers with signs of corrosion or other mechanical defects.
- It is not allowed to centrifuge highly corrosive substances which may cause material impairment and lower mechanical properties of the rotor and round carriers.
- It isn’t allowed to use rotors and accessories not admitted by the manufacturer. Let to use commercial glass and plastic test tubes, which are destined for centrifuging in this laboratory centrifuge. One should absolutely not use poor-quality elements. Cracking of glass vessels and test tubes could result in dangerous vibration of the centrifuge.
- It is not allowed to carry out centrifugation with the rotor caps taken off or not driven tight.
References
http://trishul.sci.gu.edu.au/courses/7204BPS/Centrifugation_Lecture_2008.pdf
http://www.biologydiscussion.com/biochemistry/centrifugation/centrifuge-introduction-types-uses-and-other-details-with-diagram/12489













No comments