X-Ray Spectroscopy - Principle, Protocol, Applications, Advantages, Limitations
X‐ray spectroscopy is a powerful method giving the insight into the chemical and electronic structure of studied samples. Since twentieth century, it has been extensively used in a plethora or research fields starting from solid‐state physics and followed by chemical and environmental sciences, archeological and art research, as well as biological and health studies.
X-ray spectroscopy is an excellent method to determine the
structure of a compound. However, this technique requires the availability of a
compound as a single crystal. Most chemists find this process very tedious,
time consuming and it requires a skillful hand. In the event when other
spectral methods fail to reveal a compound's identity, X-ray spectroscopy is
the method of choice for structural determination where the other parameters
such as bond lengths and bond angles are also determined. However, advances in
technology have made it possible to have an NMR spectrum complete in as little
as one minute.
As in X-ray spectrometry, there is a need to reduce the
particle size to below the level at which it will adversely affect the
diffracted intensities. In practice, this means to about the 5 μm level. Since
the action of grinding the sample can at times induce phase changes, it is
important that the grinding action is not too vigorous. This can be achieved by
slow grinding in an agate ball mill with an inert liquid medium such as
cyclohexane. This helps to keep the temperature down and also acts as a
grinding aid.
Calibration samples and samples for analysis should be
treated in the same way. The ground sample should be introduced into the sample
holder of the diffractometer using the ‘back-loading’ method which reduces the
effects of preferred orientation.
Most X-rays have a wavelength ranging from 0.01 to 10
nanometers, corresponding to frequencies in the range 30 petahertz to 30
exahertz (3×1016 Hz to 3×1019 Hz) and energies in the range 100 eV to 100 keV,
produced by the deceleration of high-energy electrons.
What is X-Ray Spectroscopy?
X-ray spectroscopy is a general term for several
spectroscopic techniques for the characterization of materials by using x-ray
excitation.
X-ray spectroscopy is a technique that detects and measures
photons, or particles of light, that have wavelengths in the X-ray portion of
the electromagnetic spectrum. It's used to help scientists understand the
chemical and elemental properties of an object.
There are several different X-ray spectroscopy methods that
are used in many disciplines of science and technology, including archaeology,
astronomy and engineering. These methods can be used independently or together
to create a more complete picture of the material or object being analyzed.
Principle of X-Ray Spectroscopy
- XRF works on methods involving interactions between electron beams and x-rays with samples.
- It is made possible by the behavior of atoms when they interact with radiation.
- When materials are excited with high-energy, short wavelength radiation (e.g., X-rays), they can become ionized.
- When an electron from the inner shell of an atom is excited by the energy of a photon, it moves to a higher energy level.
- When it returns to the low energy level, the energy which it previously gained by the excitation is emitted as a photon which has a wavelength that is characteristic for the element (there could be several characteristic wavelengths per element).
- Thus atomic X-rays emitted during electronic transitions to the inner shell states in atoms of modest atomic number.
- These X-rays since have characteristic energies related to the atomic number, and each element therefore has a characteristic X-ray spectrum which can be used to identify the element.
Working of X-Ray Spectroscopy
- An XRF spectrometer works because if a sample is illuminated by an intense X-ray beam, known as the incident beam, some of the energy is scattered, but some is also absorbed within the sample in a manner that depends on its chemistry.
- The incident X-ray beam is typically produced from a Rh target, although W, Mo, Cr and others can also be used, depending on the application.
- When x-ray hits sample, the sample emits x-rays along a spectrum of wavelengths characteristic of the type of atoms present.
- If a sample has many elements present, the use of a Wavelength Dispersive Spectrometer allows the separation of a complex emitted X-ray spectrum into characteristic wavelengths for each element present.
- Various types of detectors used to measure intensity of emitted radiation.
- The intensity of the energy measured by these detectors is proportional to the abundance of the element in the sample.
- The exact value for each element is derived from standards from prior analyses from other techniques.
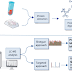
Instrumentation of X-Ray Spectroscopy
Components for X-ray spectroscopy are:
- X-ray generating equipment (X-ray tube)
- Collimator
- Monochromators
- Detectors
A. X-ray generating equipment (X-ray tube)
- X-rays can be generated by an X-ray tube.
- X-rays tube is a vacuum tube that uses a high voltage to accelerate the electrons released by a hot cathode to a high velocity.
- The high velocity electrons collide with a metal target, the anode, creating the X-rays.
B. Collimators
- A collimator is a device that narrows a beam of particles or waves.
- Narrow mean to cause the directions of motion to become more aligned in a specific direction (i.e., collimated or parallel).
- Collimation is achieved by using a series of closely spaced ,parallel metal plates or by a bundle of tubes ,0.5 or less in diameter.
C. Monochromator
- Monochromator crystals partially polarize an unpolarized X-ray beam.
- The main goal of a monochromator is to separate and transmit a narrow portion of the optical signal chosen from a wider range of wavelengths available at the input.
Types of Monochromator
- Metallic Filter Type
- Diffraction grating type
D. X-ray Detectors
The most commonly employed detectors include:
- Solid State Detectors
- Scintillation Detectors
Solid-State Detectors
- The charge carriers in semiconductor are electrons and holes.
- Radiation incident upon the semiconducting junction produces electron-hole pairs as it passes through it.
- Electrons and holes are swept away under the influence of the electric field, and the proper electronics can collect the charge in a pulse.
Scintillation detectors
Scintillation detectors consist of a scintillator and a device, such as a PMT (Photomultiplier tubes), that converts the light into an electrical signal.
- It consists of an evacuated glass tube containing a photocathode, typically 10 to 12 electrodes called dynodes, and an anode.
- Electrons emitted by the photocathode are attracted to the first dynode and are accelerated to kinetic energies equal to the potential difference between the photocathode and the first dynode.
- When these electrons strike the first dynode, about 5 electrons are ejected from the dynode for each electron hitting it.
- These electrons are attracted to the second dynode, and so on, finally reaching the anode.
- Total amplification of the PMT is the product of the individual amplifications at each dynode.
- Amplification can be adjusted by changing the voltage applied to the PMT.
Applications of X-Ray Spectroscopy
X-ray spectrometry is used in a wide range of applications, including:
- Research in igneous, sedimentary, and metamorphic petrology
- Soil surveys
- Mining (e.g., measuring the grade of ore)
- Cement production
- Ceramic and glass manufacturing
- Metallurgy (e.g., quality control)
- Environmental studies (e.g., analyses of particulate matter on air filters)
- Petroleum industry (e.g., sulfur content of crude oils and petroleum products)
- Field analysis in geological and environmental studies (using portable, hand-held XRF spectrometers)
Advantages of X-Ray Spectroscopy
- X-ray spectroscopy is an excellent method to determine the structure of a compound.
- In the event when other spectral methods fail to reveal a compound’s identity, X-ray spectroscopy is the method of choice for structural determination where the other parameters such as bond lengths and bond angles are also determined.
Limitations of X-Ray Spectroscopy
- The technique requires the availability of a compound as a single crystal.
- Most chemists find this process very tedious, time consuming and it requires a skillful hand.
References
https://slideplayer.com/slide/5770713/
http://instructor.physics.lsa.umich.edu/adv-labs/X-Ray_Spectroscopy/x_ray_spectroscopy_v2.pdf
https://www.iucr.org/__data/assets/pdf_file/0013/733/chap16.pdf
http://www.issp.ac.ru/ebooks/books/open/X-Ray_Spectroscopy.pdf



.png)







No comments