Ion Exchange Chromatography - Definition, Principle, Procedure, Applications, Advantages, Limitations
Ion exchange chromatography represents a versatile analytical technique that separates ions and polar molecules in a solution (mobile phase) based on their affinities for an ion exchanger (stationary phase). It is widely used as a preparative technique for Atomic Absorption Spectroscopy (AAS), Multiple Collector Inductively Coupled Plasma Mass Spectrometry (MC-ICP-MS), and Thermal Ionization Mass Spectrometry (TIMS) or techniques using electrical conductivity or UV detectors.
Ion exchange chromatography is a type of liquid
chromatography in which ions are separated by their adsorption onto a support
that contains fixed charges on its surface. This method relies on the
interaction (or exchange) of ions in the sample or mobile phase with fixed
ionic groups of the opposite charge that are bound to the support and act as
the stationary phase. Depending on the charge of the groups that make up the
stationary phase, the types of ions that bind to the column may be either
cations (ie, positively charged ions) or anions (ie, negatively charged ions).
These two methods are referred to as cation-exchange chromatography and
anion-exchange chromatography, respectively.
Supports for cation-exchange chromatography contain
negatively charged functional groups. These groups may be the conjugate bases
of strong acids, such as sulfonate ions that are formed by the deprotonation of
sulfonic acid, or the conjugate bases of weak acids, such as those produced
from carboxyl or carboxymethyl groups. The supports used in anion-exchange
chromatography are usually the conjugate acids of strongly basic quaternary
amines, such as triethylaminoethyl groups, or the conjugate acids of weak
bases, such as aminoethyl or diethylaminoethyl groups. Supports that can be
modified to contain these charged groups for use in Ion exchange chromatography
include silica and polystyrene, as well as carbohydrate-based materials such as
agarose, dextran, or cellulose. The carbohydrate-based supports are
particularly useful in preparative work with biological agents, which can have
strong binding to materials such as underivatized silica or polystyrene. The
large pore size of supports such as agarose also makes these materials valuable
in separations involving biological macromolecules such as proteins and nucleic
acids.
A strong mobile phase in Ion exchange chromatography is usually a mobile phase that contains a high concentration of competing ions. The presence of these competing ions will make it more difficult for a charged analyte to bind to the fixed charges that act as the stationary phase. A weak mobile phase in Ion exchange chromatography is one that contains few or no competing ions or that otherwise promotes binding by charged analytes to the column. Changing the competing ion concentration is the most common approach for adjusting the retention of analyte ions in Ion exchange chromatography. The retention of ions in this method also may be affected by (1) pH, (2) the type of competing ion used, (3) the type of fixed charges used as the stationary phase, and (4) the density of these fixed charges on the support. Many stationary phases in Ion exchange chromatography can exhibit mixed-mode retention through a combination of coulombic interactions and adsorption. As an example, ion-exchange resins that are used for amino acid analysis are able to separate amino acids with virtually the same charge because of differences in the adsorption of these amino acids onto the stationary phase.
What is Ion Exchange Chromatography?
Ion exchange chromatography (or ion chromatography) is a
process that allows the separation of ions and polar molecules based on their
affinity to ion exchangers.
The principle of separation is thus by reversible exchange
of ions between the target ions present in the sample solution to the ions
present on ion exchangers.
In this process, two types of exchangers i.e., cationic and anionic exchangers can be used.
- Cationic exchangers possess negatively charged group, and these will attract positively charged cations. These exchangers are also called “Acidic ion exchange” materials, because their negative charges result from the ionization of acidic group.
- Anionic exchangers have positively charged groups that will attract negatively charged anions. These are also called “Basic ion exchange” materials.
- Ion exchange chromatography is most often performed in the form of column chromatography. However, there are also thin-layer chromatographic methods that work basically based on the principle of ion exchange.
Working Principle of ion exchange chromatography
This form of chromatography relies on the attraction between oppositely charged stationary phase, known as an ion exchanger, and analyte.
- The ion exchangers basically contain charged groups covalently linked to the surface of an insoluble matrix.
- The charged groups of the matrix can be positively or negatively charged.
- When suspended in an aqueous solution, the charged groups of the matrix will be surrounded by ions of the opposite charge.
- In this “ion cloud”, ions can be reversibly exchanged without changing the nature and the properties of the matrix.
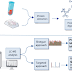
Instrumentation or component of ion exchange chromatography
Typical IC instrumentation includes: pump, injector, column,
suppressor, detector and recorder or data system.
Pump
The IC pump is considered to be one of the most important
components in the system which has to provide a continuous constant flow of the
eluent through the IC injector, column, and detector.
Injector
Sample introduction can be accomplished in various ways. The
simplest method is to use an injection valve. Liquid samples may be injected
directly and solid samples need only to be dissolved in an appropriate solvent.
Injectors should provide the possibility of injecting the liquid sample within
the range of 0.1 to 100 ml of volume with high reproducibility and under high
pressure (up to the 4000 psi).
Columns
Depending on its ultimate use and area of application, the
column material may be stainless steel, titanium, glass or an inert plastic
such as PEEK. The column can vary in diameter from about 2mm to 5 cm and in
length from 3 cm to 50 cm depending on whether it is to be used for normal
analytical purposes, microanalysis, high speed analyses or preparative work.
Guard column is placed anterior to the separating column.
This serves as a protective factor that prolongs the life and usefulness of the
separation column. They are dependable columns designed to filter or remove
particles that clog the separation column
Suppressor
The suppressor reduces the background conductivity of the
chemicals used to elute samples from the ion-exchange column which improves the
conductivity measurement of the ions being tested. IC suppressors are
membrane-based devices which are designed to convert the ionic eluent to water
as a means of enhancing the sensitivity.
Detectors
Electrical conductivity detector is commonly use.
Data system
In routine analysis, where no automation is needed, a
pre-programmed computing integrator may be sufficient. For higher control
levels, a more intelligent device is necessary, such as a data station or
minicomputer.
Procedure of ion exchange chromatography
- Ion exchange separations are carried out mainly in columns packed with an ion-exchanger.
- These ionic exchangers are commercially available. They are made up of styrene and divinyl benzene. Example. DEAE-cellulose is an anionic exchanger, CM-cellulose is a cationic exchanger.
- The choice of the exchanger depends upon the charge of particle to be separated. To separate anions “Anionic exchanger” is used, to separate cations “Cationic exchanger” is used.
- First the column is filled with ion exchanger then the sample is applied followed by the buffer. The tris-buffer, pyridine buffer, acetate buffer, citrate and phosphate buffers are widely used.
- The particles which have high affinity for ion exchanger will come down the column along with buffers.
- In next step using corresponding buffer separates the tightly bound particles.
- Then these particles are analyzed spectroscopically.
Applications of ion exchange chromatography
- An important use of ion-exchange chromatography is in the routine analysis of amino acid mixtures.
- The 20 principal amino acids from blood serum or from the hydrolysis of proteins are separated and used in clinical diagnosis.
- This is most effective method for water purification. Complete deionization of water (or) a non-electrolyte solution is performed by exchanging solute cations for hydrogen ions and solute anions for hydroxyl ions. This is usually achieved by method is used for softening of drinking water.
- In the analysis of products of hydrolysis of nucleic acids. In this way, information is gained about the structure of these molecules and how it relates to their biological function as carriers of hereditary information.
- Chelating resins are used to collect trace metals from seawater.
- To analyze lunar rocks and rare trace elements on Earth.
Advantages of ion exchange chromatography
- It is one of the most efficient methods for the separation of charged particles.
- It can be used for almost any kind of charged molecule including large proteins, small nucleotides and amino acids.
- Ion exchange is used for both analytical and preparative purposes in the laboratory, the analytical uses being the more common.
- Inorganic ions also can be separated by ion-exchange chromatography.
Limitations of ion exchange chromatography
- Only charged molecules can be separated.
- Buffer Requirement
References
Wilson, K., Walker, J. (2018). Principles and Techniques of
Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New
York.
https://www.biochemden.com/ion-exchange-chromatography/











No comments