Electron Spin Resonance (ESR) - Principle, Applications
Electron paramagnetic resonance (EPR) spectroscopy, also
known as electron spin resonance spectroscopy (ESR), utilizes absorption of
microwave radiation by unpaired electrons in a magnetic field. The interaction
between the unpaired electron(s) and nearby magnetic nuclei helps identify
paramagnetic species and can provide information about the motion of the
molecule and the local polarity, pH, viscosity, concentration, and
accessibility to other paramagnetic species. This mini-review discusses the
fundamental underpinnings of EPR needed to correctly interpret EPR spectra. We
describe various types of EPR spectra encountered by chemical engineers, and
use application examples drawn from the chemical engineering literature to
illustrate the information available from the technique. Few chemical
engineering departments or even chemistry departments have EPR instruments,
which contributes to the significant barrier that prevents this being adopted
as a routine measurement technique. However, in 2016 and 2017, Web of Science
indexed 7000 articles that applied EPR spectroscopy. A bibliometric map
categorized the keywords in four categories based on co-occurrences: magnetic
properties, films, and luminescence; crystal structure, complexes, and ligands;
nanoparticles, oxidation, and degradation; and, systems, radicals, and H2O2.
Electron Spin Resonance (ESR) also known as Electron Magnetic Resonance (EMR) or Electron Paramagnetic Resonance (EPR) is a branch of absorption spectroscopy in which radiations having frequency in the microwave region (0.04 – 25 cm) is absorbed by paramagnetic substances to induce transitions between magnetic energy levels of electrons with unpaired spins.
ESR is based on the fact that atoms, ions, molecules or
molecular fragments which have an odd number of electrons exhibit
characteristic magnetic properties. An electron has a spin and due to spin
there is magnetic moment.
Since its discovery in 1944 by E.K. Zavoisky, EPR spectroscopy has been exploited as a very sensitive and informative technique for the investigation of different kinds of paramagnetic species in solid or liquid states.
Principle of Electron Spin Resonance (ESR)
The phenomenon of electron spin resonance (ESR) is based on
the fact that an electron is a charged particle. It spins around its axis and
this causes it to act like a tiny bar magnet. When a molecule or compound with
an unpaired electron is placed in a strong magnetic field The spin of the
unpaired electron can align in two different ways creating two spin states ms =
± ½.
The alignment can either be along the direction (parellel)
to the magnetic field which corresponds to the lower energy state ms = – ½
Opposite (antiparallel) to the direction of the applied magnetic field ms = + ½
The two alignments have different energies and this
difference in energy lifts the degeneracy of the electron spin states. The
energy difference is given by:
∆ E = E+ – E- = hv = gmßB
Where,
h = Planck’s constant (6.626 x 10-34 J s-1)
v = the frequency of radiation
ß = Bohr magneton (9.274 x 10-24 J T-1) B = strength of the
magnetic field in Tesla
g = the g-factor which is a unit less measurement of the intrinsic
magnetic moment of the electron, and its value for a free electron is 2.0023.
An unpaired electron can move between the two energy levels
by either absorbing or emitting a photon of energy {\displaystyle h\nu } hv
such that the resonance condition, hv = ∆ E, is obeyed. This leads to the
fundamental equation of EPR spectroscopy.
Working of Electron Spin Resonance (ESR)
- Although the equation permits a large combination of frequency and magnetic field values, the great majority of EPR measurements are made with microwaves in the 9000–10000 MHz (9–10 GHz) region.
- EPR spectra can be generated mostly by keeping the photon frequency fixed while varying the magnetic field incident on a sample.
- A collection of paramagnetic centers, such as free radicals, is exposed to microwaves at a fixed frequency.
- By increasing an external magnetic field, the gap between the and energy states is widened until it matches the energy of the microwaves.
- At this point the unpaired electrons can move between their two spin states. Since there typically are more electrons in the lower state, due to the Maxwell–Boltzmann distribution, there is a net absorption of energy.
- It is this absorption that is monitored and converted into a spectrum.
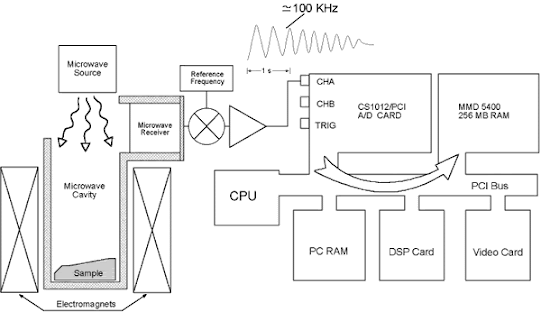
Instrumentation of Electron Spin Resonance (ESR)
KLYSTRONS
- Klystron tube acts as the source of radiation.
- It is stabilized against temperature fluctuation by immersion in an oil bath or by forced air cooling.
- The frequency of the monochromatic radiation is determined by the voltage applied to klystron.
- It is kept a fixed frequency by an automatic control circuit and provides a power output of about 300 milli watts.
WAVE GUIDE OR WAVEMETER
- The wave meter is put in between the oscillator and attenuator.
- To know the frequency of microwaves produced by klystron oscillator.
- The wave meter is usually calibrated in frequency unit (megahertz) instead of wavelength.
- Wave guide is a hollow, rectangular brass tube. It is used to convey the wave radiation to the sample and crystal.
ATTENUATORS
- The power propagated down the wave guide may be continuously decreased by inserting a piece of resistive material into the wave guide. This piece is called variable attenuator.
- It is used in varying the power of the sample from the full power of klystron to one attenuated by a force 100 or more.
ISOLATORS
- It’s device which minimizes vibrations in the frequency of microwaves produced by klystron oscillator.
- Isolators are used to prevent the reflection of microwave power back into the radiation source.
- It is a strip of ferrite material which allows micro waves in one direction only.
- It also stabilizes the frequency of the klystron.
SAMPLE CAVITIES
- The heart of the ESR spectrometer is the resonant cavity containing the sample.
- Rectangular TE120 cavity and cylindrical TE011 cavity have widely been used.
- In most of the ESR spectrometers, dual sample cavities are generally used This is done for simultaneous observation of a sample and a reference material.
- Since magnetic field interacts with the sample to cause spin resonance the sample is placed where the intensity of magnetic field is greatest.
COUPLERS AND MATCHING SCREWS
- The various components of the micro wave assembly to be coupled together by making use of irises or slots of various sizes.
CRYSTAL DETECTORS
- Silicon crystal detectors, which converts the radiation in D.C has widely been used as a detector of microwave radiation.
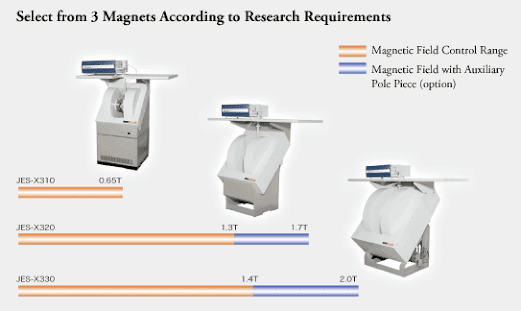
MAGNET SYSTEM
- The resonant cavity is placed between the poles pieces of an electromagnet.
- The field should be stable and uniform over the sample volume.
- The stability of field is achieved by energizing the magnet with a highly regulated power supply.
- The ESR spectrum is recorded by slowly varying the magnetic field through the resonance condense by sweeping the current supplied to the magnet by the power supply.
MODULATION COIL
- The modulation of the signal at a frequency consistent with good signal noise ratio in the crystal detector is accomplished by a small alternating variation of the magnetic field.
- The variation is produced by supplying an A.C. signal to modulation coil oriented with respect the sample in the same direction as the magnetic field.
- If the modulation is of low frequency (400 cycles/sec or less), the coils can be mounted outside the cavity and even on the magnet pole pieces.
- For higher modulation frequencies, modulation coils must be mounted inside the resonant cavity or cavities constructed of a non-metallic material e.g., Quartz with a tin silvered plating.
DISPLAY DEVICES
- In order to observe the signal a system is connected different devices can be used.
Applications of Electron Spin Resonance (ESR)
- ESR spectrometry is one of the main methods to study transition metal containing metalloproteins.
- To determine the rate of catalysis
- To know about the active site geometry
- To study denaturation and protein folding
- In studies relating to enzyme-ligand interaction
- In Biological Systems
- Study of Free Radicals
- Spin Labels
- Study of Inorganic Compounds
- Reaction Velocities & Mechanisms
- Study of naturally occurring substances such as minerals with transition elements, minerals with defects (e.g; quartz), Hemoglobin (Fe), Petroleum, Coal, Rubber etc.
- Conducting Electrons
References
https://www.jeol.co.jp/en/products/esr/basics.html
https://www.slideshare.net/kamleshpatade7/electron-spin-resonance-spectrometry
http://www.life.illinois.edu/crofts/pdf_files/Dikanov_Crofts_EPR_review_Chapter-3-2006.pdf









No comments