Affinity Chromatography - Principle, Parts, Procedure, Applications
The earliest forms of affinity chromatography were actually developed by immunochemists. In 1951 Campbell, Luescher and Lerman developed antigen–cellulose columns for use in isolating specific antibodies, a separation that could not be achieved unless one could take advantage of the affinity of the antibody for its specific antigen. The conjugation of an antigen to a solid matrix was called an immunoadsorbent and will be further described in the section on immunoadsorbents. In 1968, Cuatrecasas, Wilchek and Anfinsen applied this concept to the isolation of enzymes and introduced the term affinity chromatography. Application of the technique exploded not only because the generality of the approach became clear, but also because a new solid support based on agarose with appropriate chemistry for coupling many types of ligands was introduced. Since then this technique has had extensive application in all aspects of the biological sciences.
Recently, the use of affinity chromatography has been
integrated with high-pressure liquid chromatography (HPLC) systems to combine
the specificity of affinity chromatography with the speed and sensitivity of
HPLC. This has been extended to include high-pressure immunoaffinity
chromatography. One area that has been under-exploited is the use of specific
antibodies to isolate biological materials such as growth factors that are
produced at low concentrations in cell culture.
Affinity chromatography can be defined as a liquid
chromatographic method in which a biological agent or biomimetic ligand is used
for the selective retention of complementary compounds. This form of liquid
chromatography was originally used by Starkenstein in 1910 for the purification
of amylase through the use of starch as a solid support. This method continued
to slowly develop over the next 50 years. However, it was not until the 1960s
that suitable supports like beaded agarose, as developed by Hjerten, became
available as well as relatively simple immobilization techniques for these
supports. An important advancement in this latter area was a report in 1967 by
Axen, Porath, and Ernback in which the cyanogen bromide method for protein and
peptide immobilization was first reported. This approach was then used in 1968
by Cuatrecasas, Anfinsen, and Wilchek to purify enzymes through the use of
immobilized enzyme inhibitors. It was also at this time that the term ‘affinity
chromatography’ was proposed to describe this technique.
Affinity chromatography is relatively simple to perform and
is a powerful tool for the separation of biological macromolecules. The high
selectively of this approach often allows single-step purification strategies
to be developed, even when working with dilute and highly complex mixtures.
This simplicity and the variety of ligands that can be used with this approach
have made it an important tool in process-scale separations. However, modern
affinity chromatography also plays an important role in the analysis and study
of biological systems. For instance, most forms of chiral liquid
chromatography, such as those using immobilized cyclodextrins or serum
proteins, can be considered a subcategory of affinity chromatography.
What is Affinity Chromatography?
- Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
- It is a separation technique in which a mobile phase carrying a mixture is caused to move in contact with a selectively absorbent stationary phase.
- Affinity chromatography is a type of liquid chromatography for the separation, purification or specific analysis of sample components.
- It utilizes the reversible biological interaction or molecular recognition called affinity which refers to the attracting forced exerted in different degrees between atoms which cause them to remain in combination.
Example: Enzyme with an inhibitor, antigen with an antibody,
etc.
- It was discovered by Pedro Cuatrecasas and Meir Wilcheck.
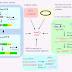
Principle of Affinity Chromatography
The stationary phase consists of a support medium, on which
the substrate (ligand) is bound covalently, in such a way that the reactive
groups that are essential for binding of the target molecule are exposed.
As the crude mixture of the substances is passed through the
chromatography column, substances with binding site for the immobilized
substrate bind to the stationary phase, while all other substances is eluted in
the void volume of the column.
Once the other substances are eluted, the bound target molecules can be eluted by methods such as including a competing ligand in the mobile phase or changing the pH, ionic strength or polarity conditions.
Components of Affinity Chromatography
1. Matrix
- The matrix is an inert support to which a ligand can be directly or indirectly coupled.
- In order to for the matrix to be effective it must have certain characters:
- Matrix should be chemically and physically inert.
- It must be insoluble in solvents and buffers employed in the process
- It must be chemically and mechanically stable.
- It must be easily coupled to a ligand or spacer arm onto which the ligand can be attached.
- It must exhibit good flow properties and have a relatively large surface area for attachment.
- The most useful matrix materials are agarose and polyacrylamide.
2. Spacer arm
- It is used to improve binding between ligand and target molecule by overcoming any effects of steric hindrance.
3. Ligand
- It refers to the molecule that binds reversibly to a specific target molecule.
- The ligand can be selected only after the nature of the macromolecule to be isolated is known.
- When a hormone receptor protein is to be purified by affinity chromatography, the hormone itself is an ideal candidate for the ligand.
- For antibody isolation, an antigen or hapten may be used as ligand.
- If an enzyme is to be purified,a substrate analog, inhibitor, cofactor, or effector may be used as a the immobilized ligand.
Steps in Affinity Chromatography
- Affinity medium is equilibrated in binding buffer.
- Sample is applied under conditions that favor specific binding of the target molecule(s) to a complementary binding substance (the ligand). Target substances bind specifically, but reversibly, to the ligand and unbound material washes through the column.
- Elution is performed specifically, using a competitive ligand, or non-specifically, by changing the pH, ionic strength or polarity. Target protein is collected in a purified, concentrated form.
- Affinity medium is re-equilibrated with binding buffer.
These events can be summarized into the following three
major steps:
1. Preparation of Column
- The column is loaded with solid support such as sepharose, agarose, cellulose etc.
- Ligand is selected according to the desired isolate.
- Spacer arm is attached between the ligand and solid support.
2. Loading of Sample
- Solution containing a mixture of substances is poured into the elution column and allowed to run at a controlled rate.
3. Elution of Ligand-Molecule Complex
- Target substance is recovered by changing conditions to favor elution of the bound molecules.
Applications of Affinity Chromatography
- Affinity chromatography is one of the most useful methods for the separation and purification of specific products.
- It is essentially a sample purification technique, used primarily for biological molecules such as proteins.
Its major application includes:
- Separation of mixture of compounds.
- Removal of impurities or in purification process.
- In enzyme assays
- Detection of substrates
- Investigation of binding sites of enzymes
- In in vitro antigen-antibody reactions
- Detection of Single Nuceotide polymorphisms and mutations in nucleic acids
Advantages of Affinity Chromatography
- High specificity
- Target molecules can be obtained in a highly pure state
- Single step purification
- The matrix can be reused rapidly.
- The matrix is a solid, can be easily washed and dried.
- Give purified product with high yield.
- Affinity chromatography can also be used to remove specific contaminants, such as proteases.
Limitations of Affinity Chromatography
- Time consuming method.
- More amounts of solvents are required which may be expensive.
- Intense labour
- Non-specific adsorption cannot be totally eliminated, it can only be minimized.
- Limited availability and high cost of immobilized ligands.
- Proteins get denatured if required pH is not adjusted.
References
Wilson, K., Walker, J. (2018). Principles and Techniques of
Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New
York.
https://www.med.unc.edu/pharm/sondeklab/files/resource-files/protein-purification-handbooks/Affinity%20chromatography.pdf











No comments